
Principal uses of strontium compounds are in pyrotechnics, for the brilliant reds in fireworks and warning flares and in greases.
Strontium has uses similar to those of calcium and barium, but it is rarely employed because of its higher cost. Finely powdered strontium metal will ignite spontaneously in air to produce both strontium oxide and strontium nitride.
Due to its extreme reactivity to air, this element always naturally occurs combined with other elements and compounds.

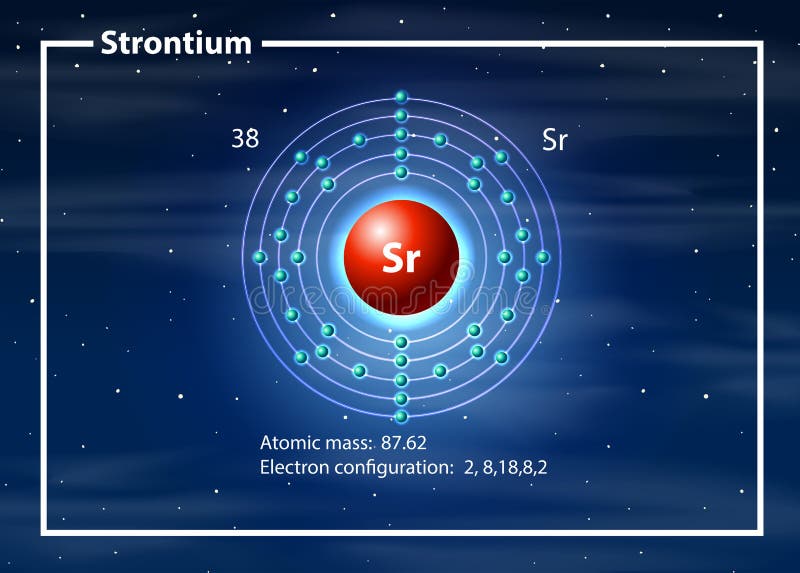
Strontium reacts vigorously with water and quickly tarnishes in air, so it must be stored out of contact with air and water. It has three allotropic crystalline forms and in its physical and chemical properties it is similar to calcium and barium. Strontium is a soft, silver-yellow, alkaline-earth metal. Strontium - Sr Chemical properties of strontium - Health effects of strontium - Environmental effects of strontium
SR ELEMENT COMMON ION FREE
Elements Common Charges 1 Charge of Hydrogenionġ+ 2 Charge of Heliumion 0 3 Charge of Lithiumionġ- 10 Charge of Neonion 0 11 Charge of Sodiumionġ- 18 Charge of Argonion 0 19 Charge of PotassiumionĢ+, 3+, 4+, 5+ 24 Charges of Chromiumions 2+, 3+,6+ 25 Charges of Manganeseions 2+, 4+, 7+ 26 Charges of Ironionsģ+ 32 Charges of Germaniumions 4-, 2+, 4+ 33 Charges of Arsenicions 3-, 3+, 5+ 34 Charges of Seleniumions 2-, 4+, 6+ 35 Charges of Bromineionsġ-, 1+, 5+ 36 Charge of Kryptonion 0 37 Charge of Rubidiumion 1+ 38 Charge of Strontiumion 2+ 39 Charge of Yttriumion 3+ 40 Charge of Zirconiumion 4+ 41 Charges of Niobiumions 3+, 5+ 42 Charges of Molybdenumions 3+, 6+ 43 Charge of Technetiumion 6+ 44 Charges of Rutheniumions 3+, 4+, 8+ 45 Charge of Rhodiumion 4+ 46 Charges of Palladiumions 2+, 4+ 47 Charge of Silverionġ+ 48 Charge of Cadmiumion 2+ 49 Charge of Indiumion 3+ 50 Charges of Tinions 2+, 4+ 51 Charges of Antimonyions 3-, 3+, 5+ 52 Charges of Telluriumions 2-, 4+, 6+ 53 Charge of Iodineionġ- 54 Charge of Xenonion 0 55 Charge of Cesiumionġ+ 56 Charge of Bariumion 2+ 57 Charge of Lanthanumion 3+ 58 Charges of Ceriumions 3+, 4+ 59 Charge of Praseodymiumion 3+ 60 Charges of Neodymiumions 3+, 4+ 61 Charge of Promethiumion 3+ 62 Charge of Samariumion 3+ 63 Charge of Europiumion 3+ 64 Charge of Gadoliniumion 3+ 65 Charges of Terbiumions 3+, 4+ 66 Charge of Dysprosiumion 3+ 67 Charge of Holmiumion 3+ 68 Charge of Erbiumion 3+ 69 Charge of Thuliumion 3+ 70 Charge of Ytterbiumion 3+ 71 Charge of Lutetiumion 3+ 72 Charge of Hafniumion 4+ 73 Charge of Tantalumion 5+ 74 Charge of Tungstenion 6+ 75 Charges of Rheniumions 2+, 4+, 6+, 7+ 76 Charges of Osmiumions 3+, 4+, 6+, 8+ 77 Charges of Iridiumions 3+, 4+, 6+ 78 Charges of Platinumions 2+, 4+, 6+ 79 Charges of Goldions 1+, 2+, 3+ 80 Charges of Mercuryions 1+, 2+ 81 Charges of Thalliumions 1+, 3+ 82 Charges of Leadions 2+, 4+ 83 Charge of Bismuthion 3+ 84 Charges of Poloniumions 2+, 4+ 85 Charge of Astatineion Unknown 86 Charge of Radonion 0 87 Charge of Franciumion Unknown 88 Charge of Radiumion 2+ 89 Charge of Actiniumion 3+ 90 Charge of Thoriumion 4+ 91 Charge of Protactiniumion 5+ 92 Charges of Uranium ions 3+, 4+, 6+ Free Gift for you: Interactive Periodic Table List of elements with their common ionic charges are mentioned below.Įlements with multiple ionic charges are also mentioned in this table. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. This electric charge generated on the ion is known as Ionic charge. Ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed).


 0 kommentar(er)
0 kommentar(er)
